About ANTHIM® (obiltoxaximab)
EFFICACY
Overview
Because it is not feasible or ethical to conduct controlled clinical trials in humans with inhalational anthrax, the efficacy of ANTHIM for the treatment of inhalational anthrax is based on efficacy studies in NZW rabbits and cynomolgus macaques. The animal efficacy studies are conducted under widely varying conditions, such that the survival rates observed in the animal studies cannot be directly compared between studies and may not reflect the rates observed in clinical practice.
Types of Studies
The efficacy of ANTHIM for treatment and prophylaxis of inhalational anthrax was studied in multiple studies in the cynomolgus macaques and NZW rabbit models of inhalational anthrax. These studies tested the efficacy of ANTHIM compared to placebo and the efficacy of ANTHIM in combination with antibacterial drugs relative to the antibacterial drugs alone.
Study Design
The animals were challenged with aerosolized Bacillus anthracis spores (Ames strain) at approximately 200xLD50 to achieve 100% mortality if untreated. In prophylaxis studies of inhalational anthrax, animals were treated prior to the development of symptoms. In treatment studies, animals were administered treatment after exhibiting clinical signs or symptoms of systemic anthrax. Cynomolgus macaques were treated at the time of a positive serum electrochemiluminescence (ECL) assay for B. anthracis PA at a mean time of approximately 40 hours post-challenge with B. anthracis. In NZW rabbit treatment studies, animals were treated after a positive ECL assay for PA or sustained elevation of body temperature above baseline, at a mean time of approximately 30 hours post-challenge. The majority of animals triggered by temperature. In some of the treatment studies assessing the effect of ANTHIM in combination with antibacterial drugs, treatment was delayed to 72 to 96 hours post-challenge. Most study animals were bacteremic and had a positive ECL assay for PA prior to treatment. Survival was assessed at 28 days post-challenge with B. anthracis in most studies.
Results
Two studies in NZW rabbit and two studies in cynomolgus macaques evaluated treatment with ANTHIM 16 mg/kg IV single dose compared to placebo in animals with systemic anthrax. Treatment with ANTHIM alone resulted in statistically significant improvement in survival relative to placebo in both species. Survival rates were 93% and 62% with ANTHIM compared to 0 or 0 placebo survivors in rabbits and 47% and 31-35% survival with ANTHIM compared to 6% or 0% placebo survival in macaques (see table below).
Survival Proportions in Monotherapy Treatment Studies of 16 mg/kg IV, All Randomized Animals Positive for Bacteremia Prior to Treatment
| Proportion of Survival at Day 28a (# survived/n) | p-valueb | 95% CIc | ||
| Placebo | ANTHIM 16 mg/kg IV | |||
| NZW Rabbits | ||||
| Study 1 | 0 (0/9) | 93% (13/14) | 0.0010 | (0.59, 1.00) |
| Study 2 | 0 (0/13) | 62% (8/13) | 0.0013 | (0.29, 0.86) |
| Cynomolgus Macaques | ||||
| Study 3 | 6 % (1/16) | 47% (7/15) | 0.0068 | (0.09, 0.68) |
| Study 4d | 0 (0/17) | 31% (5/16)
35% (6/17) |
0.0085 0.0055 |
(0.08, 0.59) (0.11, 0.62) |
IV: intravenous, CI: Confidence Interval
- aSurvival assessed 28 days after spore challenge
- bp-value is from 1-sided Boschloo Test (with Berger-Boos modification of gamma=0.001) compared to placebo
- cExact 95% confidence interval of difference in survival rates
- dANTHIM products manufactured at two different facilities were tested in two separate treatment arms.
ANTHIM administered in combination with antibacterial drugs (levofloxacin, ciprofloxacin and doxycycline) for the treatment of systemic inhalational anthrax disease resulted in higher survival outcomes than antibacterial therapy alone in multiple studies where ANTHIM and antibacterial therapy was given at various doses and treatment times (see figure below).1
Survival Rates for ANTHIM in Combination with Antibiotics vs Antibiotic Alone Treatment (All Treated Animals)
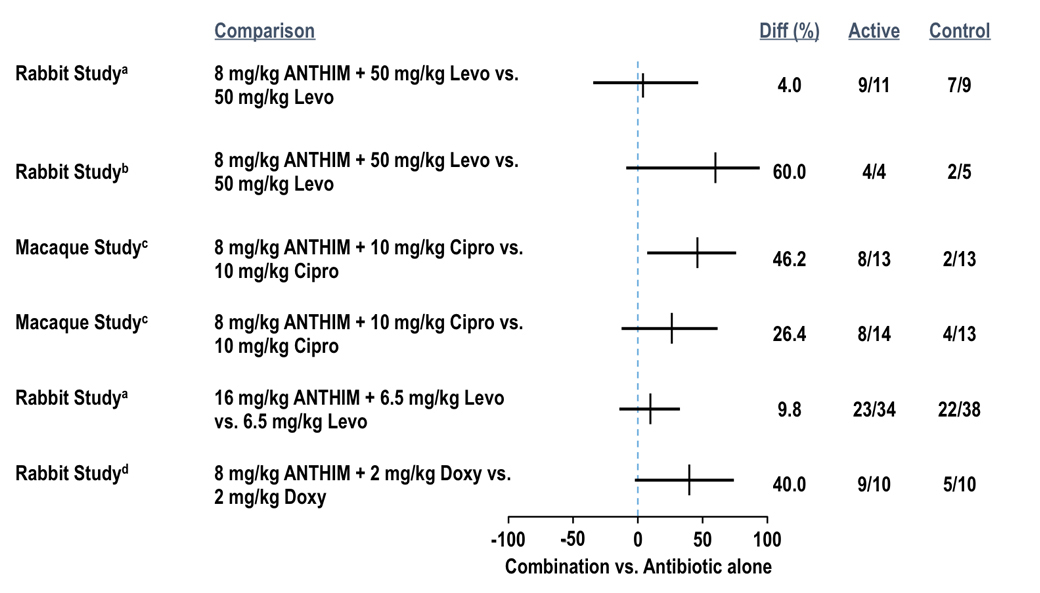
- aTreatment administered at 72 hours post-challenge
- bTreatment administered at 96 hours post-challenge
- cTreatment administered 24 hours after a positive ECL assay for PA
- dTreatment administered at the time of a positive ECL assay for PA
ANTHIM administered as prophylaxis resulted in higher survival outcomes compared to placebo in multiple studies where treatment was given at various doses and treatment times.1 In one study, cynomolgus macaques were administered ANTHIM 16 mg/kg at 18 hours, 24 hours or 36 hours after exposure. Survival was 6/6 (100%) at 18 hours, 5/6 (83%) at 24 hours, and 3/6 (50%) at 36 hours (see table below). Another cynomolgus macaque study evaluated ANTHIM 16 mg/kg administered 72, 48 or 24 hours prior to exposure. Survival was 100% at all three time points (14/14, 14/14, 15/15, respectively) at day 56 (end of study).
Survival Rates in Cynomologous Macaques in Postexposure Prophylaxis, All Challenged Animals
| Treatment Time (hrs) | Number (%) Survivala | p-valueb | 95% CIc | |
| Placebo | – | 0/6 (0%) |
– | – |
| ANTHIM 16 mg/kg | 18 | 6/6 (100%) |
0.0012 | (0.471, 1.000) |
| ANTHIM 16 mg/kg | 24 | 5/6 (83%) |
0.0042 | (0.230, 0.996) |
| ANTHIM 16 mg/kg | 36 | 3/6
(50%) |
0.0345 | (-0.037, 0.882) |
CI: Confidence Interval
- aSurvival assessed after spore challenge (28 days), except 16 mg/kg dose was assessed at 56 days.
- bp-value is from 1-sided Boschloo Test (with Berger-Boos modification of gamma=0.001) compared to control
- cExact 95% confidence interval of difference in survival rates
References
- Data on file. Elusys Therapeutics, Inc. Pine Brook, NJ.
INDICATIONS AND USAGE AND IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE
ANTHIM® (obiltoxaximab) is indicated in adult and pediatric patients for the treatment of inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs. ANTHIM is indicated for prophylaxis of inhalational anthrax due to B. anthracis when alternative therapies are not available or are not appropriate.
LIMITATIONS OF USE
- ANTHIM should only be used for prophylaxis when its benefit for prevention of inhalational anthrax outweighs the risk of serious hypersensitivity reactions and anaphylaxis.
- The effectiveness of ANTHIM is based solely on efficacy studies in animal models of inhalational anthrax. It is not ethical or feasible to conduct controlled clinical trials with intentional exposure of humans to anthrax.
- Safety and PK of ANTHIM have been studied in adult healthy volunteers. There have been no studies of safety or PK of ANTHIM in the pediatric population. A population PK approach was used to derive intravenous infusion dosing regimens that are predicted to provide pediatric patients with exposure comparable to the observed exposure in adults.
- ANTHIM binds to the protective antigen (PA) component of B. anthracis toxin; it does not have direct antibacterial activity. ANTHIM is not expected to cross the blood-brain barrier and does not prevent or treat meningitis. ANTHIM should be used in combination with appropriate antibacterial drugs.
IMPORTANT SAFETY INFORMATION
WARNING: HYPERSENSITIVITY and ANAPHYLAXIS
Hypersensitivity and anaphylaxis have been reported during the intravenous infusion of ANTHIM. Due to the risk of hypersensitivity and anaphylaxis, ANTHIM should be administered in monitored settings by personnel trained and equipped to manage anaphylaxis. Monitor individuals who receive ANTHIM closely for signs and symptoms of hypersensitivity reactions throughout the infusion and for a period of time after administration. Stop ANTHIM infusion immediately and treat appropriately if hypersensitivity or anaphylaxis occurs.
WARNINGS AND PRECAUTIONS
Hypersensitivity reactions were the most common adverse reactions in the safety trials of ANTHIM, occurring in 34/320 healthy subjects (10.6%). Three (0.9%) cases of anaphylaxis occurred during or immediately after the infusion. In clinical trials, manifestations of anaphylaxis were rash/urticaria, cough, dyspnea, cyanosis, postural dizziness and chest discomfort. ANTHIM infusion was discontinued in 8 (2.5%) subjects due to hypersensitivity or anaphylaxis. The adverse reactions reported in these 8 subjects included urticaria, rash, cough, pruritus, dizziness, throat irritation, dysphonia, dyspnea and chest discomfort. The remaining subjects with hypersensitivity had predominantly skin-related symptoms such as pruritus and rash, and 6 subjects reported cough.
Premedication with diphenhydramine is recommended prior to administration of ANTHIM. Diphenhydramine premedication does not prevent anaphylaxis, and may mask or delay onset of symptoms of hypersensitivity.
ADVERSE REACTIONS
The safety of ANTHIM has been studied only in healthy volunteers. It has not been studied in patients with inhalational anthrax. The most frequently reported adverse reactions (occurred in >1.5% of healthy subjects) were headache, pruritus, infections of the upper respiratory tract, cough, vessel puncture site bruise, infusion site swelling, urticaria, nasal congestion, infusion site pain, and pain in extremity.
USE IN SPECIFIC POPULATIONS
Pregnancy
No adequate and well-controlled studies in pregnant women were conducted. Because animal reproduction studies are not always predictive of human response, ANTHIM should be used during pregnancy only if clearly needed.
Pediatric Use
There have been no studies of the safety or PK of ANTHIM in the pediatric population.
To see the complete prescribing information for ANTHIM, click here.
